Submitted by Prof. C.J. Pickard on Wed, 12/10/2016 - 19:24
Research has revealed the fate of the vital elements carbon and oxygen when subjected to the extremes of compression encountered in giant planets and stars.
The centres of planets and stars are well hidden from us. Squeezing samples between two diamonds (diamond anvil cell experiments) have allowed some materials to be examined at pressures approaching those found at the centre of the Earth. But Jupiter, Saturn, Uranus, Neptune and a host of recently discovered exoplanets are much larger, and gravitational forces generate considerably greater pressures at their cores.
The laws of physics that govern the behaviour of matter, even to these extreme pressures, were established in the early 20th century - those of quantum mechanics and special relativity. But we are far from understanding all the consequences of these rules, and scientists have been consistently surprised by what they observe. Nature is notoriously more inventive than the most imaginative theoretical physicist.
So how might we hope to predict what is going on in these hidden away parts of the universe, in the centre of massive planets and stars? The approach taken by Chris Pickard and Richard Needs has been to perform a thorough computational search through the possible configurations that the atoms may take, using a reliable quantum mechanical description of the electrons that bind them together.
Dubbed Ab Initio Random Structure Searching, following its introduction in 2006 it has been responsible for numerous predictions, some of which already confirmed experimentally, and others being actively investigated by experimenters.
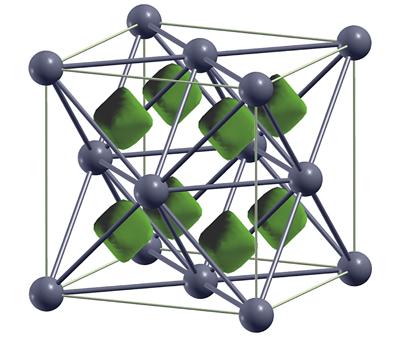
In two papers, published in the same issue of Physical Review Letters, the nature of carbon and oxygen under extreme compression has been explored. Four new phases of carbon were found, with one of them (pictured right, top) being found to be an “electride” - or a binary ionic compound, the electrons taking on the role of the anions. Oxygen molecules were found to survive to much higher pressures than expected, followed by a complicated series of electronic transitions including a metal-insulator transition as the oxygen polymerises (pictured right, bottom).
Thermodynamically Stable Phases of Carbon at Multiterapascal Pressures. Miguel Martinez-Canales, Chris J. Pickard and Richard J. Needs. Physical Review Letters, 108, 045704 (2012)
Persistence and Eventual Demise of Oxygen Molecules at Terapascal Pressures. Jian Sun, Miguel Martinez-Canales, Dennis D. Klug, Chris J. Pickard and Richard J. Needs. Physical Review Letters, 108, 045503 (2012)

