Submitted by Administrator on Wed, 08/08/2018 - 12:23
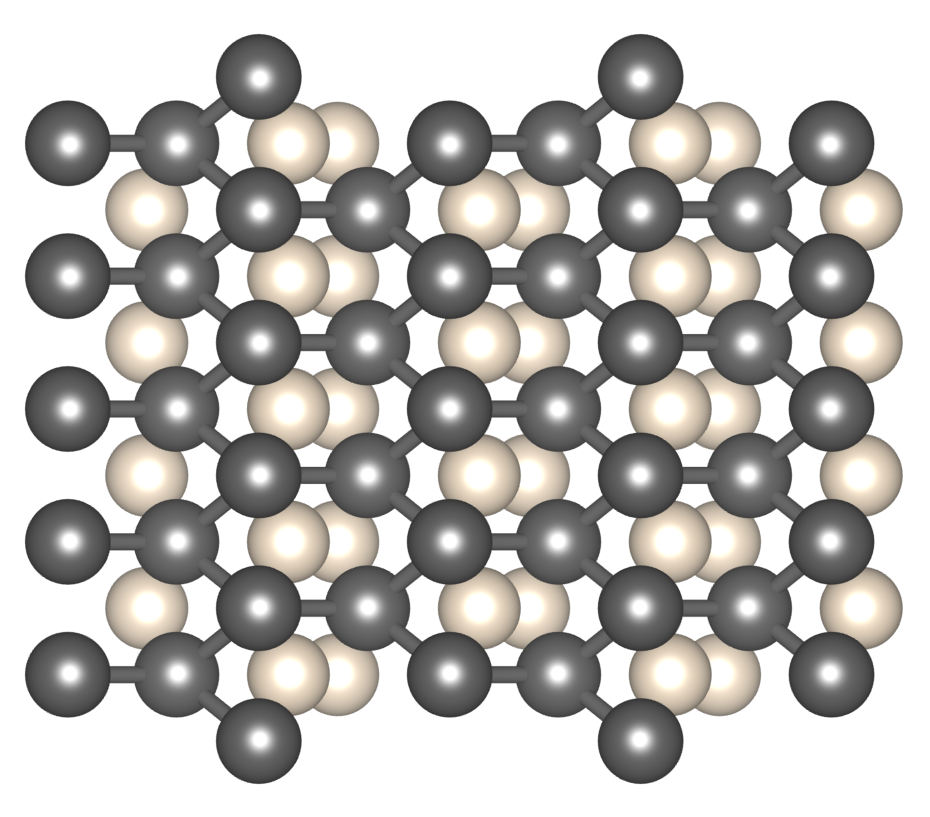
Helium is the second most abundant element in the universe, but as the most noble gas it is extremely chemically inert. It strongly resists forming compounds. Iron is also highly abundant. With its excellent material properties, especially when alloyed with other elements, iron has played a central role in human development. It also forms an important part of planets and other astronomical bodies. The Earth’s core is largely iron. Iron is also expected to be found in the centre of large exoplanets, and white dwarf stars.
A recent theoretical study, conducted by researchers at the Universities of Cambridge and Edinburgh, asked - might iron and helium react under some conditions? The surprising answer is yes, and at pressures to be found within the Solar System - in the core of Jupiter and possibly Saturn. By computationally searching through randomly generated compositions and structures, and optimising them to their lowest quantum mechanical enthalpy, compounds of iron and helium were discovered which are much more stable than iron or helium separately. Future models of the planets and stars should treat helium as a compound forming element.
Helium-Iron Compounds at Terapascal Pressures
Bartomeu Monserrat, Miguel Martinez-Canales, Richard J. Needs, and Chris J. Pickard
Physical Review Letters, 121, 015301 – Published 3 July 2018

